Clinical Data Management (CDM) is an important stage in Clinical Research, which leads to generation of high-quality, reliable, and statistically sound data from clinical trials. Clinical data management helps collection, integration and availability of data at appropriate quality and cost.
Duration of the course: The course duration is 6 months In- class sessions = 6 months & Internship = 2 months(Optional). Is Internship provided ? Yes, the internship will be provided at the end of the course for a period of two months.
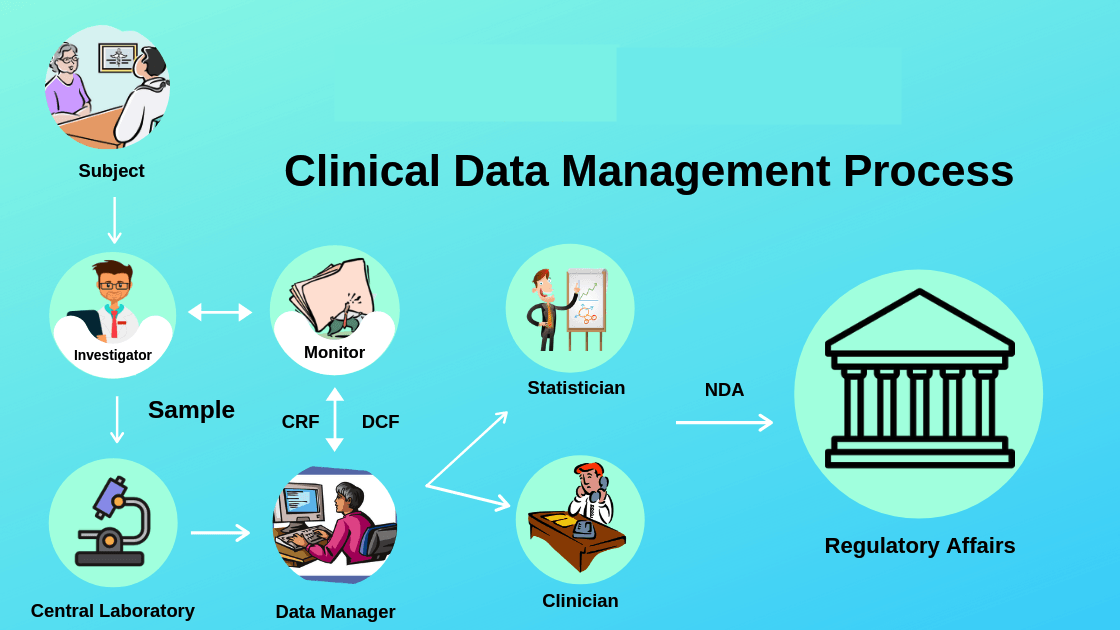
Clinical Data Management involves data privacy, data storage, record keeping, data protection, destroying the data and sharing and reporting the data. The process of collecting and managing research data is done in accordance with the standards set by the regulatory authority. Data management plays an important role in the drug development process and evaluation of accurate drugs. CDM professionals are taught to ensure standards for improving data quality.
Various Clinical Data Management courses have been developed with the primary objectives of introduction to the clinical data management process, understanding of tools, and management of clinical data. Due to rapid advancements in clinical trials and research, this field offers huge scope to students.
Clinical Data Management Highlights The Clinical Data Management course makes students learn the importance of data in clinical trials. They learn different aspects of effective management of data under the Clinical Data Management course. Candidates can check the table below to learn more about the Clinical Data Management course details.

